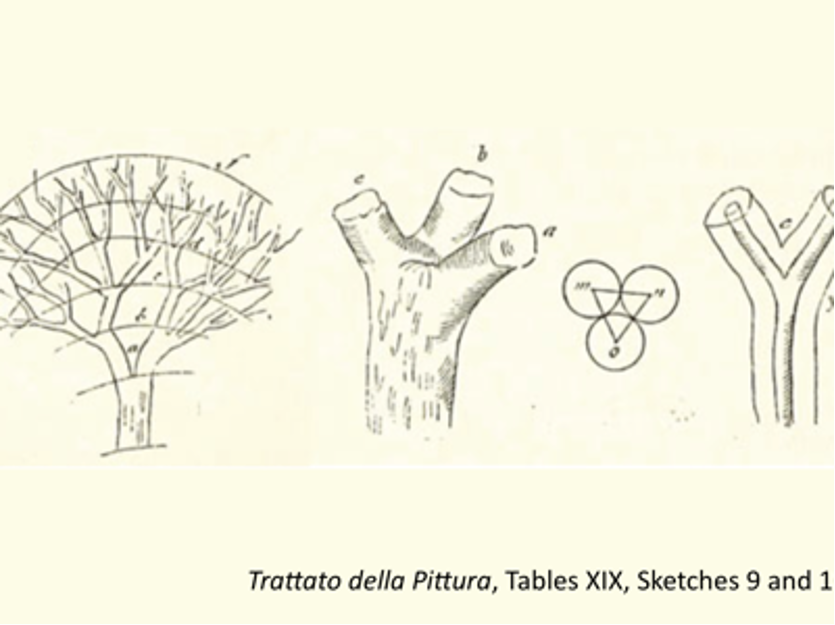
Leonardo da Vinci was used to pull brilliant ideas out of nowhere, with a depth of thinking that is still astounding today. Even if some of his scientific and artistic intuitions were just wrong, so many others came so close to the reality that it makes one wonder, how he could see so acutely and so far-reaching, just by painstaking observation and with very little maths. In a typical example, in his Trattato della Pittura (Treatise on Painting), he put forward a sheer intuition about the bifurcation of fluid flow in the trees: Ogni biforcazione di rami insieme giunta ricompone la grossezza del ramo che con essa si congiunge […] e questo nasce perché l’umore del più grosso si divide secondo i rami. [Each bifurcation when joined together gives the same thickness of the branch from which it stems (…) and this because the fluid of the thicker branch is divided according to the thinner ones.] In simpler words, according to Leonardo, if a tree’s branches were to be folded upward and squeezed together, the tree would look like one big trunk with the same thickness from top to bottom: a “law” that is very difficult to verify experimentally, but apparently comes close to the results of complex mathematical models [ see: Phys Rev Lett 107, 258101 (2011) ].
Probably, the best explanation of this (fully heuristic) principle is not due to internal tree hydraulics, but rather to mechanical reasons, that is the need to support high bending stress from winds; which seems reasonable, when considering that the internal tubules and capillaries occupy but a minuscule fraction of the tree volume, while the rest is dense, sturdy and elastic wood. However, the bold connection that Leonardo made with the fluid flow inside the tree branches, likely originated from another observation collected from his notebooks, for which he is usually credited with the first observation of capillarity and the definition of the ascending and descending flows in the tree structure.
In modern terms, we know that capillarity is the result of the contact forces at the fluid interfaces, mainly governed by the combination of surface tension 𝛴, the size of the capillary R, and the contact angle 𝜃 (related to the curvature). By equating the gravity acting on the mass of the fluid and the adhesion force at the triple (gas/fluid/wall) interface, the equilibrium height reached by the liquid in the capillary is given by the so-called Jurin equation, h=2𝛴cos𝜃/ρgR ; by putting the values for a water-air interface, it is h≃1.48 10-5/R. This well-known figure is at the basis, among other things, of the upwards capillary flow of the sap (a mixture of water and nutrients) in the minuscule channels of the plant stems, or the trunk of trees.
Some time ago, I made a toy model of tree capillarity for the 2nd-year students of physics. The typical diameters of tree capillaries is about 10-30 μm, or R≃5-15 μm. Then, the maximum height h reachable by capillary pressure only, is of the order of a few meters. This value can be enough for a plant, but it does not explain how we can have trees growing up to tens of meters high. One structural solution that nature adopted is branching. In my extremely simplified theoretical description of a tree, I’d put together many single channels of radius R and height h1, parallel to each other. To calculate what is the effect of branching the tree at its extremity, consider each single channel as branching into N smaller channels of radius r over a height h2, so that the maximum total height could be H=h1+h2. To conserve the same quantity of fluid transported by capillarity in these two sections of the “tree”, we impose the conservation of the transverse cross section, Nπr2=πR2, that is just the Leonardo’s principle above. Then, the capillarity force acting in each channel in the lower trunk plus the N branches of the thinner bifurcations is easily shown to be proportional to (1+N1/2); on the other hand, the total gravity force on the fluid is proportional to (1+h2/h1). It so turns out that, in order to maximise the height, such a weird-shaped tree should have N → ∞, but to minimise the gravity, h2 → 0. The practical considerations from such a naively simplified model of capillary feeding, is that a tree should typically develop a large number of branches N in its upper part, but no branches in its lower part; and that the upper branched part should be much less important in size than the trunk, h2≪h1. In fact, this is just what it is qualitatively observed in nature for a large variety of plants and trees.
In practice, the capillarity mechanism can operate only until mechanical equilibrium is attained, after which the transport of sap towards the top of the tree stops. Actually, it is the evaporation of water from the leaves surface, combined with the difference in saline concentrations in the soil at the level of the roots, which provides an effective pressure (depression from evaporation, plus osmotic pressure from salt concentrations). This extra pressure constantly keeps the system out of equilibrium, thereby permitting the continuous flux of sap and nutrients from the bottom to the top. Water climbing to a height h by capillarity must overcome a pressure difference equal to ∆P = ρgh, which keeps going until the maximum height is attained. After that, water from the top must be removed, to make room for more water climbing up. For a tree with typical height of about 20-30 m the work done by capillarity force is mgh = (0.018 kg/mol)(9.81 m s–2)(30 m) = 5.3 J per mole of water; the latent heat of evaporation of water being 44 kJ/mol (supplied by the heat of the Sun), it is way larger than the work done by capillarity. Therefore, more energy is necessary to remove the water from the top than it is necessary to bring it up there, in full obedience of the Second Principle of thermodynamics. If this were not the case, the tree would be creating a “Perpetual Motion of the Second Kind”, namely a machine that spontaneously converts thermal energy into mechanical work…
It is also instructive to compute the pressure corresponding to a capillary of a few nm diameter, that is: ρgh=ρg(1.48×10–5)/R =1000*9,81*1.48×10–5/10–9=1,5 kbar. At this size, just a few water molecules can fill the capillary: at the lowest limit of a width accommodating just one water molecule, the pressure gets above 5 kbar. And this last observation leads us to the paper that I wanted to present you in this first Sunday of 2021, and which ended up stimulating all the above reflections.
In a work published in Nature just three weeks ago [ vol. 588, p. 250 (2020) ], Andrej Geim and coworkers (yes… “that” Geim) created nanoscale capillaries by sandwiching strips of graphene between atomically-flat crystals of mica. Some channels were just one carbon layer wide, allowing only one single layer of water molecules to pass through. Using AFM, Geim and colleagues imaged the capillaries, as they filled up with water. Their data showed that capillary condensation still follows the macroscopic (Kelvin’s) equations even in these nanometric structures.
Such a result came as a big surprise, since the properties of confined water drastically change at the nanoscale: the short-range ordering of molecules should become distinctly discrete and layered, displaying an oscillating density profile, up to at least a thickness of 1-1.5 nm. For capillary condensation to occur at relative humidity well below 100%, Lord Kelvin dictates that R must be comparable to (2𝛴/kBTρ) ≈ 1 nm (with the 𝛴 and ρ of water). But at this length, the notions of R and contact angle 𝜃 are indeed vanishing, and the Kelvin equation should be modified, since it originally describes just bulk water. What Geim et al. surprisingly found is that, instead, the contact forces at the nanoscale (essentially Van der Waals, for graphene layers) adjust so as to cancel out the effects of molecular ordering, by means of elastic fluctuations of the walls equal and opposite to the water density fluctuations.
Given the above values of pressure, this finding appears now quite reasonable: at an equivalent mechanical pressure of 5 kbar, a surface bending of a few % can be obtained in the elastic graphene layers, and much more so in natural materials (such as lignin or cellulose) which have bending moduli about 10 times smaller.
But, look at the sketch in Fig.1c, and the plots in Fig.1e of their paper: you will notice that the graphene surface is bent inwards, even at the highest relative humidity. This is an important detail that is often overlooked in common explanations of capillarity phenomena: the pressure inside the capillary is actually below that of free air, that is the ∆P is negative, and the pressure jump at the curved interface is balanced by the surface tension times the curvature. At least two interesting consequences follow thereafter.
One, is that the boiling point of water inside the capillary is lowered with respect to STP conditions: already in a capillary of a few microns, the pressure difference is such that the boiling point is below room temperature. For example, water in the higher branches of the trees is likely to be vapor, instead of liquid – a supersaturated vapor indeed.
Second, a “negative” pressure is usually interpreted as relative to atmospheric, but we mean it to be always positive in absolute value (which is certainly true for a gas). However, imagine what happens if we do a capillarity experiment in vacuum: we can get to an absolute negative pressure! And now, try to recompute the strange thermodynamics of this liquid inside the capillary (for example, it can be simultaneously superheated and supercooled…)
I know, you are starting to get tired of reading by now. So, just for the most curious among you, I suggest to have a look at the Journal of Non-equilibrium Thermodynamics, vol. 23 p. 351 (1998)…